Buffer Maker - easy buffer design
If you are not satisfied with buffers present in Buffer Maker database, you can always try to design your own buffers, that will better suit your needs.
Buffer Maker comes with a database of weak and strong acids and reagents. You can add your own substances at any time. This is described in details in program tutorial. For now let's assume you want to check whether mixture of citric acid and sodium phosphate (both present in program database) will behave as a buffer, and if so - what could be its range of application.
After opening new document you can select both reagents - citric acid and sodium phosphate - from the reagent database. You do it clicking on the database... buttons in the reagent frames and selecting reagents from the list. This is how the reagent database dialog window looks like:

After selecting reagent from the list you click on the Use button. You may also double click reagent name on the list, which will close the dialog and copy selected reagent to main buffer view.
When both reagents are selected, there is one important thing to do left. You have to select which substance will be treated as main component. Main component is the one whose concentration is entered as buffer concentration. If you select citric acid as main component, Buffer Maker will calculate amounts of mixed reagents keeping sum of concentrations of all forms of citric acid equal to the entered value. You may select phosphoric as main component as well. You may even select sodium hydroxide - although it is not explicitly used in buffer preparation it is added in the neutralized form as sodium phosphate.

Now you can check how this mixture behaves as a buffer. The most important information is presented on the buffer capacity plot. It clearly shows that mixture of citric acid will resist pH changes in the pH range 2.5-7.5, with maximum somewhere between pH 6 and pH 7, than capacity goes quickly down.
Using the same approach you can compare buffer capacity plots for buffer made mixing sodium citrate and phosphoric acid:

Or even solution of both acids neutralized with sodium hydroxide to requested pH:

While in each case the same conjugate acids and bases are present, they are in different ratios and thus their buffering ability at different pH is different. At the same time you can be sure you have very similar composition of solutions in the wide pH range, which means you don't have to worry about side effects of different buffers. With Buffer Maker you can easily check which buffer will best suit your needs. It can be extremely helpful when designing series of experiments in different pH.
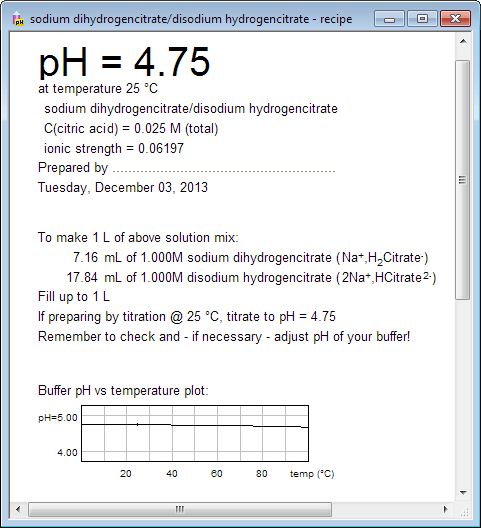
You can also check how your buffer will behave in different temperatures. There is no pH vs temperature plot in the main document view, but such plot is displayed in the recipe view. Switch to recipe view using either menu, or tool bar, or pressing Ctrl-R keys.